Radiation therapy plays an important role in cancer management. Today 45% of all cancer patients can be cured. About one half of them are cured by radiation therapy applied alone or in combination with surgery or chemotherapy. The clinical experience accumulated in decades shows that, to be efficient, the radiation treatment must be delivered with a high physical selectivity. At present, electron linear accelerators are the primary equipment of a modern radiotherapy department, and are used to irradiate a large proportion of the patients for at least part of the treatment. Photon beams of about 6-20 MV have, in general, a sufficient penetration in the tissues to treat most of the tumors with an adequate physical selectivity. A combination of several beams adequately oriented allows the radiation-oncologist to deliver the prescribed dose to the "target volume" (tumor) without exceeding the tolerance of the surrounding normal tissues. Conformation therapy, which needs well-equipped and well-staffed centers, further improves the physical selectivity of the treatment, and offers definitive advantages at least for some tumor types and/or locations. Finally, modern linear accelerators also allow to apply electron beam therapy. Electron beam therapy is suitable for the treatment of superficial lesions and is also the best available irradiation technique for Intra-Operative-Radiation-Therapy (IORT).
Electron linear accelerators today constitute the core of the equipment of a modern radiation therapy department. Nowadays, the majority of the patients referred to a radiation therapy department are treated with a linear accelerator for at least part of their treatment. It is likely that this will remain true for the foreseeable future. Linear accelerators thus play, and will keep playing, a significant role in cancer management in general and are responsible for the therapeutic success obtained in treatment of many tumors. To illustrate the situation, Table 1 gives the number of linear accelerators at present used in France for radiotherapy applications (their type and energy). The numbers of other radiotherapy units are also given for comparison [1].
| Total * | |||||||
| 4-6 MV | Orion 5 | Siemens (Mevatron) | 36 | ||||
| Philips (SL 75/5) | (+5) | ||||||
| Clinac 600 C | |||||||
| 10 MV | Neptune 10 | 29 | |||||
| Saturne 1M | (-1) | ||||||
| 15 MV | Saturne 15 | Siemens (Mevatron MD) | 51 | ||||
| Saturme 1 | Philips (SL 18) | (+17) | |||||
| Saturne 41 | |||||||
| 20 MV | Saturne 20 | Philips (SL 75/20) | 31 | ||||
| Saturne 11 | Clinac 2100C | (-17) | |||||
| Saturne 42 | |||||||
| 25-40 MV | Sagittaire 32 MV | 15 | |||||
| Sagittaire 40 MV | (-2) | ||||||
| Saturne 25 MV | |||||||
| 20-25 MV | Saturne 111 | 4 | |||||
| 25 MV | Saturne 43 | Philips (SL 75/25) | 56 | ||||
| Siemens (Mevetron KD2) | (+12) | ||||||
| Total Linear Accelerators | 222 | ||||||
| (+14) | |||||||
| Cobalt Units | |||||||
| Betatron | |||||||
| Hadron Therapy | |||||||
| Cyclotron: neutron therapy (Orléans) | |||||||
| Cyclotron: neutron + protontherapy (Nice) | |||||||
| Synchrotron: protontherapy (Saclay) | |||||||
| * The differences since 01.01.1994 are given (+) or (-) | |||||||
Cancer has an increasing impact on the death rates in the Western world, as well as in the developing countries. For example, in 1990, 890,000 deaths were attributed to cancer in the countries of the European Union and there were 1,385,000 new cancer cases diagnosed in that year alone [2]. These estimates exclude non-melanoma skin cancer, which, although a rare cause of death, nevertheless demands medical care. Even with the current prevention programs, the numbers will further increase within the next two decades. Similar figures have been published for North America.
Today, at the first consultation, approximately 65% of the patients have apparently localized tumors. About 2/3 of these are cured either by surgery, radiotherapy, or a combination of both treatments. In this group of patients with probable but unproved subclinical metastatic disease, chemotherapy used as an adjutant treatment may prolong life and maybe cure some additional patients.
Among the other 35% of patients arriving at the first consultation with already inoperable or metastatic disease, only about 5% will be cured by combined treatment including chemotherapy and immunotherapy as well as radiotherapy and/or surgery (Table 2). Although there is promising progress in the field of medical oncology, this cure rate is largely limited to solid paediatric tumors, leukemias, lymphomas, and testicular tumors. These tumors represent only about 5% of all cancers seen in a general population [3,4]. Although these percentages are useful as an indication of the contribution of the different techniques to cancer cure, they will become progressively less relevant to the extent that combination of these different techniques is more and more successfully applied.
| Patients with localised tumor at the first consultation (65%) | ||||||||
|
||||||||
| Patients with inoperable or metastatic disease at first consultation (35%) | ||||||||
| Total | ||||||||
It is axiomatic that one must control the local disease if one aims ultimately at curing any cancer patient. In fact, it has been shown that 1/3 of the patients who die of cancer has uncontrolled local disease. If local failure could be reduced by 50%, one could expect a 10-15% improvement in cure rate [5].
Surgical techniques have already reached a very high level. Further improvement will be seen in a reduction of mutilating procedures (limb-sparing operation, breast-conserving therapy, reduction of colostomies, and urinary diversions). On the other hand, wider excisions are still foreseeable as a result of safer anaesthesiology, intensive care support, and improvement in reconstructive surgery.
Furthermore, the combination, on a wider scale, of surgery with irradiation will help to increase the local control rate. In that respect, Fletcher (M.D. Anderson Hospital, Houston) has shown that, after radical surgery, doses of 50 Gy given over 5 weeks are able to eradicate expected occult infestation ("subclinical disease") in the lymphatic nodes for cancer of the breast, upper respiratory and digestive track and some pelvic tumors, in more than 90% of the cases. Doses of 50 Gy do not exceed the tolerance limit of many normal tissues.
Today, about 70% of all cancer patients are referred to a radiation therapy department, either for a radical treatment (aiming at a local control) or after surgery (also aiming at a local control) or for a palliative treatment often in combination with chemotherapy (e.g., a painful bone metastasis) [6].
Soon after the discovery of x-rays by Röntgen in 1895 and of radium by Marie Curie in 1898, it became evident that ionizing radiations could sterilize malignant tumors and thus cure cancer patients. It also became rapidly evident that, above a certain dose level, x-rays induce damage to the normal tissues they traverse.
The probability of local control of a tumor increases with radiation absorbed dose. Therefore, a sufficiently high dose should be delivered to the whole volume(s) of tissues invaded by malignant cells (i.e., "target volume(s)"). This goal must be reached without inducing severe and irreversible sequelae in the surrounding normal tissues [7]. A first approach to reach this goal is the improvement of the physical selectivity.
2.1 Physical selectivity of a therapeutic irradiation
The physical selectivity of a therapeutic irradiation is defined as the ratio of the dose to the "target volume" (or tumor cells) relative to the dose to the surrounding normal tissues. It can be improved by varying the nature and energy of the radiation beams and their arrangement (number and orientation of the beams, and their size and shape).
Today, with modern techniques, most critical normal tissues such as brain, eyes, spinal cord, kidneys, liver, etc. may be completely avoided or at least irradiated at levels well below tolerance (except, of course, in special cases depending on the tumor location: e.g. the normal brain is the normal tissue at risk when a brain tumor is treated, similarly the spinal cord is the normal tissue at risk when some cervical or mediastinal tumors are treated, etc.).
The physical selectivity is a specific advantage of radiation therapy compared to chemotherapy in which all the tissues in the body are exposed to the toxic drug. The real situation is however more complex: outside the limits of the visible tumor, there is in general some "subclinical" involvement. A larger tissue volume must then be irradiated: not only the "gross tumor volume" itself but also a surrounding "safety margin" and, in some cases, the regional lymph node areas (see these definitions in ICRU Report 50. [8]).
2.2 Improvement of the differential effect. A problem for the radiobiologist
As defined above, the safety margin surrounding the gross tumor volume consists primarily of normal tissues and, only for a small proportion of invading cancer cells. Because of the presence of these cells, the safety margin should be irradiated in principle to the highest possible dose to prevent a local recurrence, but the tolerance of the normal tissues included in the safety margin limits the dose which can be prescribed.
The difficulty cannot be solved by improving the physical selectivity of the irradiation; it requires an improvement of the differential effect, and this brings us from the field of physics to the field of radiobiology. Improvement of the differential effect implies, for a given (physical) dose, increasing the effect on the cancer cell population and/or reducing the effects on the normal tissues [9].
Historically the first, and presently the most efficient method for improving the differential effect was the fractionation of the dose, as initiated in 1919 by Regaud, Ferroux and Coutard at the Foundation Curie in Paris. Today, five daily fractions of 2 Gy each per week are used as the conventional fractionation for most treatments.
Combination of radiation with radiosensitizers is another method to improve the differential effect. Drugs such as misonidazole are used to sensitize selectively hypoxic cancer cells. Other drugs, such as actinomycin D, bleomycin, adriamycin, 5-fluorouracil, and, more recently, cis-platinum have been used as radiosensitizers in the treatment of oesophagus, lung, or intestinal tumors. Besides any synergistic effects, radiation and drugs have several complementary actions which further justify their therapeutic association.
A third possibility for improving the differential effect is to replace x-rays (and other low-LET radiations) by high-LET radiations (ICRU Report 45 [8]). High-LET radiations bring a benefit for well-differentiated, slowly growing tumors and/or for tumors containing a large proportion of hypoxic cancer cells [10].
2.3 Accuracy required in radiation therapy
Whatever the improvement in the radiobiological differential effect, the doses which are needed to control a malignant tumor are often of the same order of magnitude as the tolerance doses for the normal tissues. In addition, radiobiological and clinical evidence indicates that the dose-effect relations for tumor control are steep [11]. For some tumors, a dose variation of a few percent can modify significantly the observed local control rate. The dose-effect relations are even steeper for normal tissue complications. For these two reasons, accurate dosimetry is needed, and, in 1976, the ICRU made the following recommendations: "the available evidence for certain types of tumors points to the need for an accuracy of + 5% in the delivery of an absorbed dose to a target volume if the eradication of primary tumor is sought" (ICRU Report 24 [8]). More recently (1987), Mijnheer et al. [11] recommended an accuracy in absorbed dose delivery of 3.5% (one standard deviation, for the dose at the specification point, for radical treatment).
3.1 Current "cross-fire" techniques with photon beams
An acceptable dose distribution can generally not be achieved with only a single photon beam. The normal tissues in front of the target volume receive too high a dose, and the normal tissues behind the target volume also receive a significant dose. Therefore, the "cross-fire" technique is currently used, where several beams intersect at the level of the target volume. As a result, the surrounding normal tissues at risk are irradiated only by one beam. The number and the orientation of the beams are selected by the radiation-oncologist; it is a matter of judgement and experience to select the optimal beam arrangement for each particular patient.
As an example, the beam arrangement and the resulting dose distribution for the treatment of a breast cancer is presented in Fig. 1.
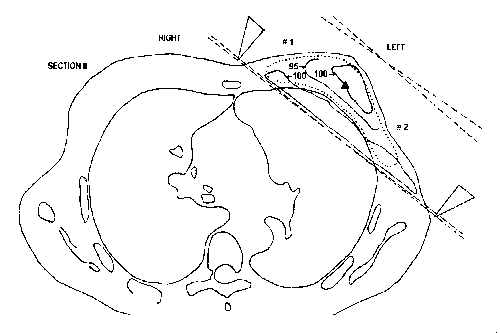
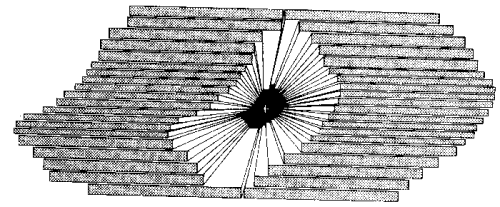
The linear accelerators are generally equipped with a variable collimator which consists of two pairs of jaws which can be moved continuously towards each other or apart from each other, defining square or rectangular beams of any size needed [12]. Recently, more and more modern linear accelerators are becoming equipped with a multileaf collimator which consists of two sets of 20 leaves which can be moved independently allowing the application of irregularly-shaped beams better adapted to the complex shape of the target volume (Fig. 2)[13].
3.2 New developements and trends in photon beam therapy
3.2.1 Stereotactic radiosurgery.
Stereotactic radiosurgery is used mainly for the treatment of
lesions limited in size and located in the head (e.g., tumors
of the base of the skull). By using a large number of narrow beams
intersecting at the level of the lesion or by using arc therapy,
high doses can be delivered to the lesion while keeping the dose
to the surrounding normal structures relatively low. This procedure
requires accurate localization of the lesion, accurate dose computation,
and accurate beam/patient positioning [14].
3.2.1 Conformation Therapy.
"3-D Conformation Radiation Therapy" may be defined as the process by which the high-dose volume is tailored to the target volume while delivering low dose to the non-target tissues of the patient. This process involves several tasks and procedures including: 1) patient fixation; 2) specification of target volume(s) and organs at risk; 3) dose prescription; 4) selection and design of beam number, shape and orientation in 3-D; 5) 3-D dose calculation; 6) plan evaluation; 7) computer-controlled dose delivery; 8) treatment verification.
3-D Conformation Radiation Therapy is clearly a new era for radiation oncology and will lead to substantially improved treatment planning and dose-delivery techniques (pp. 1-16, in [15]).
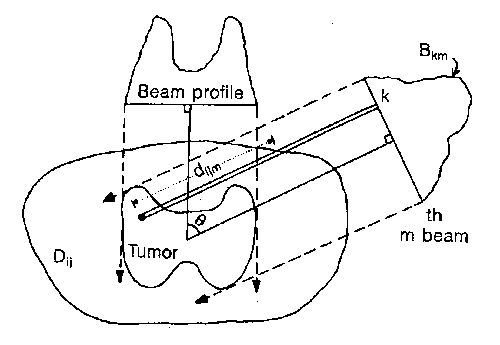
Different methods can be used in order to match the high-dose volume with the shape of the target volume. As an example, figure 3 presents an application of intensity modulation for achieving conformal isodose distribution to a concave target volume. Actually, intensity modulation can be accomplished with compensating filters (cerrobend blocks), although the manual changing of blocks can be very time consuming. When large numbers of beams are used, it is far more practical to use multi-leaf-collimator-contoured beams.
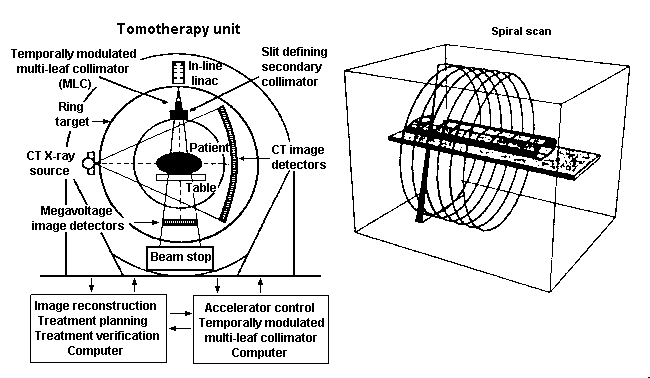
Another approach which deserves special attention is "tomotherapy" which is illustrated on figure 4 (after Mackie). A small linear accelerator is mounted on the CT-like ring gantry to deliver tomotherapy ("slice therapy"). During irradiation, the patient is moved longitudinally through a slit-collimated rotating fan beam. The intensity modulation is accomplished by moving multiple independent leaves across the slit opening. The leaves are either fully open or fully closed during most of the treatment. A megavoltage image detector is mounted opposite to the linac for treatment verification. In addition, a diagnostic x-ray tube and an array of detectors mounted on the same gantry provide CT capability to verify the position before treatment.
3.3 Electron beam therapy
Besides photon-beam therapy, modern linear accelerators allow electron-beam therapy to be applied, For that purpose, in the accelerator head, the target is removed and the beam of accelerated electrons is directly oriented towards the patient and the target volume [12].
The penetration of electron beams in the tissues is much shallower than that of the x-ray beams and, in addition, can be adjusted by varying the energy of the incident electrons. Therefore, electron-beam therapy is used to treat superficial or semi-deep-seated tumors extending (close) to the skin surface. Beyond the depth of the maximum, the dose falls off rapidly. Treatment energies range from about 4 to 20-25 MeV, but some accelerators reach higher energies up to 35 MeV. The 50 MeV electron beam produced by the race-track microtron opens new perspectives in electron beam therapy (ICRU Report 35 [8]).
Electron beams are used for 10-25% of the patients referred to
the radiation therapy department, this proportion varying from
country to country and from centre to centre depending on the
local treatment policy. Electrons are often used in combination
with photon beams (e.g., as a boost against the residual tumor).
Two specific applications of the electron beams deserve to be
mentioned.
3.3.1 Total skin electron therapy
A skin cancer, mycosis fungoides, is most often treated with total skin electron irradiation. The aim of the basic treatment is to irradiate the total skin envelope as homogeneously as possible [16]. The depth of the lesions suitable for this type of treatment varies with the stage and type of disease and/or the body surface. This may lead to the use of different beam penetrations. When tumorous lesions are present, there may be a need for a special boost and/or shielding.
The maximum depth of the target volume varies from approximately
5 to 15 mm in most of the lesions. For the most frequent indications,
with localized and even generalized plaques, the target volume
is located within the first 5 mm. Infiltrated plaques, ulcerations,
and tumorous lesions justify an individual estimate of the thickness
of the lesions whenever possible.
3.3.2 Intra-operative radiation therapy (IORT)
With this technique, electron beams are used to deliver a large single-dose fraction during surgical procedure to a well defined target volume. The dimensions of the target volume are defined accurately by the surgeon and the radiation oncologist together. During the procedure, mobile radiosensitive tissues are displaced out of the beam using localizers, in order to decrease normal tissue toxicity.
The purpose of the procedure is usually to treat presumed subclinical disease after macroscopically radical resection. The exact shape, size and location of the target volume can thus only be defined during surgery.
In conclusion, an optimal use of the available modern linear accelerators, in particular conformation therapy, will improve the outcome of the radiotherapy treatments in some groups of patients. The side effects and complications will certainly be reduced. Optimization of the treatments with linear accelerators will be facilitated by further improvement in their technical performance and reliability. In addition, several companies have designed specific types of linear accelerators for specific purposes (e.g. stereotactic radiosurgery).
Finally, the techniques of stereotactic radiosurgery and conformation therapy described above can be combined with the introduction of new radiation qualities (e.g. protons, neutrons, heavy ions). The benefit of both approaches could then be added. Application of neutron, proton and heavy-ion beams using conformation therapy techniques has actually been achieved in some centres for well-selected tumor types [10, 17].
[1] A.Laugier, ACRIM 1995, Annuaire de la Cancérologie/Radiothérapie et des Imageries Médicales en France, Hopital Tenon, Paris 20, 1995.
[2] European Commission, Europe against cancer, European Network of Cancer Registries, International Agency for Research on Cancer (IARC) and World Health Organization (WHO), Office for Official Publications of the European Communities, L-2985 Luxembourg, 1995.
[3] V.T. Devita, Progress in cancer management, Cancer, 51, 2401-2409, 1983.
[4] R. Doll, Are we winning the fight against cancer? An epidemiological assessment, EACR - Mühlboch Memorial Lecture, Eur. J. Cancer, Vol. 26,No.4, 500-508, 1990.
[5] H.D. Suit, Potential for improving survival rates for the cancer patient by increasing the efficacy of treatment of the primary lesion, Cancer, 50, 1227-1234, 1982.
[6] Commission of the European Communities, Concerted action: cancer treatment with light ions in Europe - EULIMA, Final Report - Part 1: General Feasibility Study; A. Wambersie, P. Chauvel, G. Gademann, J.P. Gerard, R. Sealy, Socio-economic study, pp. 2-39, CEC, rue de la Loi 200, 1049, Brussels, 1992.
[7] A. Wambersie and G.F. Whitmore, Radiation therapy, ICRU News, International Commission on Radiation Units and Measurements (ICRU), Inc., 1, 15-20, 1995.
[8] International Commission on Radiation Units and Measurements (ICRU):
ICRU, Inc., 7910 Woodmont Avenue, Suite 800, Bethesda, Maryland 20814-3095, USA.
[9] M. Tubiana, J. Dutreix and A. Wambersie, Introduction to radiobiology, Taylor & Francis, London, 1990.
[10] A. Wambersie, Neutron therapy: from radiobiological expectation to clinical reality, Radiation Protection Dosimetry, 44, 379-395, 1992.
[11] B.J. Mijnheer, J.J. Battermann and A. Wambersie, What degree of accuracy is required and can be achieved in photon and neutron therapy? Radiotherapy and Oncology, 8, 237-253, 1987.
[12] C.J. Karzmark, S. Nunan and E. Tanabe, Medical Electron Accelerators, McGraw-Hill, Inc., Health Professions Division, New York, 1993.
[13] A. Brahme: Optimal setting of multileaf collimators in stationery beam radiation therapy. Strahlentherapie und Oncologie, 164: 343-350, 1988.
[14] W. Lutz, K.R. Winston and N. Maleki: A system for stereotactic radiosurgery with a linear accelerator. Int. J. Radiation Oncology Biol.Phys., 14, 373-381, 1988.
[15] J.L. Meyer, J.A. Purdy, 3-D Conformal Radiotherapy, A New Era in the Irradiation of Cancer, Frontiers of Radiation Therapy and Oncology, Vol. 29. Ed.: J.L. Meyer, J.M. Vaeth, Karger, Basel, 1996.
[16] AAPM, American Association of Physicists in Medicine, C.J. Karzmark (ed): Total skin electron therapy; technique and dosimetry, Report #23, American Institute of Physics, New York, 1987.
[17] M. Austin-Seymour, R. Caplan, K. Russell, G. Laramore, J. Jacky, P. Wootton, S. Hummel, K. Lindsley and T. Griffin, Impact of a multileaf collimator on treatment morbidity in localized carcinoma of the prostate, Int. J. Radiation Oncology, Biol.Phys., 30, 1065-1071, 1994.