The most recent applications of hadron accelerators to tumor therapy are reviewed and the Italian Hadrontherapy Programme is presented in the framework of what is done and planned in the rest of the world.
This programme foresees three independent but coherent activities:
(i) the construction in Milano of a large National Centre for Oncological Hadrontherapy based on a 22 m diameter synchrotron, with 3 rooms for proton treatment and 1 room for ion treatment (which can control radioresistant tumours);
(ii) the construction (financed by Istituto Superiore di Sanità, Rome) of a novel 3 GHZ linac which will accelerate protons to 200 MeV. The second part of this linac can also be used as 200 MeV booster for protons accelerated to 60-70 MeV by high current cyclotrons;
(iii) a multimedia information network
(RITA) connecting the Associated Centres with the Centres having
hadron beams, so to select before any travelling the patients
to be irradiated.
Approximately 50% of the 10'000 accelerators
running in the world are devoted to medicine and/or biology [1].
About 80% of all the biomedical accelerators (4'000 in 1994) are
devoted to radiotherapy with either X-rays [2]; very few of them
are used for hadrontherapy. Hadrontherapy is discussed in this
paper with particular reference to the Italian Hadrontherapy Programme
promoted by the TERA Foundation.
Fifty years ago Bob Wilson remarked that the Bragg peak of monoenergetic protons, and of other charged hadrons, easily allows to deliver the dose with millimetric accuracy, what is now called a "conformal" treatment of deep sited tumours [3]. To reach 25 cm in soft tissues the kinetic energy of the protons has to be 200 MeV. For carbon ions, the most used hadrons after protons, one needs 4500 MeV, i.e. 375 MeV/u. Since the width of the Bragg peak of monoenergetic particles is very narrow, to irradiated thick targets the energy of the charged particles has to be modulated in time either by an absorber of variable thickness or by changing the energy of the accelerator.
With a Spread Out Bragg Peak (SOBP) of 8-10 cm, the distall fall-off of the dose takes place in 2-3 mm and the surface dose for proton (carbon ions) is typically 70% (50%) of the peak dose at 20-25 cm depth. These conditions are much more favourable than the ones of X-rays, which have a roughly exponential absorption in matter. Due to the convenient macroscopic energy distribution, a truly "conformal" therapy can be performed with only one or two directions of incidence of the hadron beam. Moreover the total energy delivered to the surrounding healthy tissues is definitely lower than in X-rays conformal radiotherapy which uses 6-12 crossed beams [2]. This allows an even larger tumour dose than in CRT for sites which are surrounded by critical tissues, as in the brain.
At the end of 1995 about 16000 patients had been treated with proton beams over the world [4] and about 150 with carbon ions at HIMAC (Heavy Ion Medical Accelerator Centre) in Japan [5]. The pioniering work done in LBL with helium ions (about 2000 patients) and neon ions (about 500 patients) has been discontinued in 1992 [6]. Nowdays carbon ions (Z=6) are considered to be better suited than helium (Z=2) and neon (Z=10) ions for the treatment of radioresistant tumours, because the stopping power (or LET=Linear Energy Transfer, which for a given velocity is proportional to Z2) is less than 100 MeV/cm at the entry point (so that in the first traversed layers they behave roughly as X-rays and protons) but is definitely larger than 100 MeV/cm in the spread out Bragg peak, which covers the tumour. Such a high LET is suited to treat radioresistant tumours, i.e. the slowly growing hypoxic tumours which are insensitive to both X-rays and protons and represent about 10% of all tumours treated with X-rays. Thus light ions, and in particular carbon ions, have the double property of a favourable dose distribution (crucial for a conformal radiotherapy) and of a large LET (necessary for the cure of radioresistant tumours).
Protontherapy of eye melanomas, requiring 60-70MeV protons, is performed in many, also European, centres. Deep seated tumours are treated in one dedicated hospital-based centre (at the Loma Linda University Centre in California [7]) and in ten centres which originally were, or still are, nuclear research centres:
CPO Orsay, GWI Uppsala,
HCL Boston, ITEP Moscow,
IUCF Indiana, JINR Dubna,
LINPh St.Petersburg, NAC Faure,
PMRC Tsukuba, PSI Villigen.
It is seen that only two of them are in the European Union (CPO and GWI). The HIMAC facility in Chiba (Japan) is fully dedicated to ion therapy [5]. Together with Loma Linda, this is the only hospital based dedicated hadrontherapy centre. As discussed below, in the next years the situation will change, in particular outside Europe.
In all these centres the lines transporting the hadron beam to the patient are fixed. In Loma Linda instead they are they consist in three 10 m high rotating isocentric gantries which allow the irradiation of the patient from any angle. The aligment procedure is long: typically 20-30 minutes.
Untill now no patient has been treated with a properly directed pencil bean (active beam spreading system). All centres use a scattered beam of hadrons properly shaped in space and energy with absorbers and collimators.
Active systems have been recently tested in PSI and GSI and will be used on patients towards the end of 1996. At PSI a compact gantry and a voxel scanning system for protons has been constructed [9], while the carbon beam of the GSI synchrotron will be used to irradiate patients with a horizontal raster scanned pencil beam [10]. In future all facilities will have active spreading systems, but medical doctors still prefer the passive method which is proven and guarantees an uniform irradiation in the transverse plane. Active system will have to be very reliable to be accepted.
In Europe (3.2 108 inhabitants) about 50% of all the tumours are irradiated with high energy photons. This corresponds to more than 600'000 new X-ray treatments per year, which implies about 60'000 new patients with radioresistant tumours, who could profit from iontherapy. To substantiate this large figure many clinical trials are needed at HIMAC, GSI and possibly other new iontherapy centres.
For protontherapy solid clinical results exist for brain, eye, spinal cord tumours and few other sites. Salivary glands, prostates and cervices have also been treated. A conservative analysis made in the framework of the TERA programme (described in Ref. [11]) concluded that about 5% of X-rays patients would profit (i.e. about 30'000 in Europe). In a study for Europe Gademan obtained much larger figures: 280'000 patients, of which about 25'000 first priority cases [12]. Similar results are contained in a recent unpublished report prepared for the National Cancer Institute by H.D. Suit and collaborators of the Massachussets General Hospital (MGH).
Since a multigantry centre can treat about 1000 patients/year (each one for 20 sessions lasting 20-30 minutes each) one conservatively concludes that in Europe there is space for at least twenty protontherapy centres aiming at improving the local control of tumours close to critical organs.
In the world by the year 2000 there will be at least four new dedicated facilities for hadrontherapy, on top of Loma Linda and HIMAC. Two of them (one in USA and the other in Japan) are based on the cyclotron designed by IBA [13]. For the Japanease centre of Kishawa (Chiba) IBA has teamed with Sumitomo. The American Centre (NPTC) will have two rotating gantries; it is being built in Boston by Mass General Hospital (MGH) and utilizes all the knowledge collected at the Harvard cyclotron. The Japanese centre is very similar; it will also be ready by 1998.
While these two centres will have only proton beams, in the Prefecture of Hyogo (Japan) a proton and ion centre is under construction and will be ready in 2001. The investement of 275 M$ includes a 50 bed hospital. Mitsubishi Electric is building the accelerator and the hightec facilities. The purpose is similar to the one of the Italian CNAO (see later).
The fouth funded hadron accelerator,
to be presented below, is a novel 3 GHz linac, which has been
designed in the framework of the Italian Hadrontherapy Programme,
initiated in 1992 by the TERA Foundation .
The Foundation was created to collect funds and employ a staff fully devoted to the Hadrontherapy Programme. In 1996 more than twenty people work fulltime on the projects of the Foundation, whose 1995 budget was about 1'300'000 kLit (1kLit = 1DM). In fall 1991 INFN (the Italian Institute for research in fundamental nuclear and subnuclear physics) decided to finance the research part of this activity; since then the support to the twelve Sections and Laboratories of INFN now working on the Programme has increased: in 1996 it is about 800'000 kLit. ENEA (Ente Nazionale per le Nuove tecnologie, l'Energia e L'Ambiente) joined the Programme in spring 1993 to contribute to the design of "compact" accelerators for protontherapy, a project led since then by Luigi Picardi of ENEA-INN, Frascati. Also in 1993 the physicists of Istituto Superiore di Sanità (the Italian National Health Institutes sited in Rome) decided to join the Hadrontherapy Programme and requested and obtained funds for the construction of a proton acceleretor.
Three Committees coordinate the research and development activities done in the framework of the Hadrontherapy Programme:
the Pathologies and Treatments Committee,
the Radiobiology Committee,
the Dosimetry and Microdosimetry Committee.
Their activities, not presented here, are common to all the projects of the Programme.
As far as the direct intervention of the TERA Foundation is concerned, the design and construction activities of the Hadrontherapy Programme are organized in three projects:
- (1) The planning and the construction of a National Centre for Oncological Hadrontherapy (CNAO), a healthcare and research structure of excellence which will be the focal point of all the hadrontherapy activities and - being equipped with proton and ion beams to be used in parallel - will be able to treat with protons about 1'000 patients per year, and at a later stage, an equal number with carbon ion beams.
- (2) The design and the construction of a certain number of Protontherapy Centres equipped with proton accelerators, of small dimensions and relatively cheap, possibly built by Italian industries; each of these will treat at least 200-300 patients a year with a proton beam and about double that number with an added treatment room. This is the "Compact" Accelerator Project PACO.
- (3) The creation of an informatics and organisational network, called RITA (Italian Network for Hadrontherapy Treatment), which will connect the Associated Centres - distributed throughout Italy (and abroad) and situated in the public oncological institutions and in private clinics - with the Centres where proton and ion beams will be made available. The specialised medical and physics staff in these Associated Centres will be able to discuss in remote through multimedia connections the clinical cases, with the experts of the Hadrontherapy Centre and those of the Protontherapy Centres by using the most modern informatics means. They will exchange diagnostics images and some of the physicians at these Associated Centres (sometimes after using conventional radiotherapies) will even be in a position to plan a successive treatment for their patients, which will then be irradiated in one of the Centres where hadron beams are available.
The first two projects are described
in the following Sections. For lack of space the RITA network
and other Italian hadrontherapy projects not under the direct
TERA responsability will not be further discussed.
From the beginning of 1992, the Foundation is engaged in the design and realization of the hadrontherapy centre CNAO based on a synchrotron which can accelerate protons to at least 250 MeV and carbon ions to at least 4500 MeV (i.e. at least 375 MeV/u). This will be a centre of excellence devoted to tumour hadrontherapy of more than one thousand patients/year, to clinical research in cancer therapy and to R&D in the fields of radiobiology and dosimetry. The first study was completed in spring 1994 and published in the form of a "Blue Book", which describes versions A and B of the Centro Nazionale di Adroterapia Oncologica (CNAO). Since the volume was much requested, a second edition of the Blue Book was distributed in 1995: "The TERA Project and the Centre for Oncological Hadrontherapy", Volumes I and II (U. Amaldi and M. Silari Eds, INFN, Frascati, 1995) [11]. Version C is described in the Addendum. G. Brianti is the Chairman of the CNAO Project Advisory Committee.
In 1995 CERN funded a small research activity (the TERA Group) formed of part-time physicists and engineers who, since then, contribute to the design of the medical synchrotron for protons and ions, which is at the hearth of the CNAO project. At the beginning of 1996 a new optimized study of such a synchroton was started at CERN under the leadership of Dr. Philip Bryant. Five TERA staff members and two doctoral students from the AUSTRON Project (Vienna) partecipate in the study, which aims at finding new optimized solutions for the synchrotron and the isocentric proton gantries. GSI (Darmstadft) - where at the end of 1996 Gerald Kraft and collaboratotors will start patient treatment with carbon ions - has the responsability for the design of the ion injector and of a gantry for carbon ions.
For a medical synchrotron the intensity of the extracted beams poses no special problem, since 1011 p/s and 3 109 ions/s are enough. The issue is the time uniformity of the spill since, due to the magnet ripples, synchrotron pulses have time structures at many frequencies; this makes the active spreading of the beams particularly difficult. At HIMAC [5] this problem has been partially solved with an accurate (to few 10-7), but costly, stabilisation of the magnet power supplies.
The new study of the medical synchrotron is thus taking time uniformity of the extracted beam, which lasts about one second, as the highest priority, as alreeady done at LEAR for much longer time scales.
The work is not yet completed, but the
main ideas behind a solution, possibly to be combined with other
methods, can be explained with reference to Fig. 1, in which the
transverse beam size is represented. Due to the unavoidable ripples
in the dipoles and quadrupoles, the resonance lines (drawn at
45o)
can be thought to oscillate continously in the directions indicated
by the arrows. When the beam is uniformly pushed to the resonance,
for instance with a betatron core which has no ripple, the movement
of the resonance lines produces a time disuniformity. To
reduce the effect the part of the beam which is closer to the
resonance can be made moving much faster, so that the extracted
beam is less sensitive to the ripples.
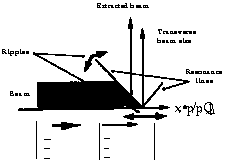
Fig. 1. The figure represents the extraction process. The arrows indicate the effect of the magnet ripples and the dashed areas the densities of the beam.
While the design of the machine goes on, TERA physicists and engineers have defined a new layout of CNAO (version D). An image is given in Fig. 2.
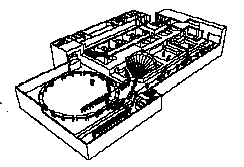
Fig. 2. The layout of CNAO. The proton/carbon
synchrotron has an average diameter of 22 m. The centre will have
three rooms for proton treatment and one with an horizontal beam
of ions. The building is extendable for constructing other therapy
rooms.
In December 1995 the TERA Foundation offered to six Hospital and oncological Institutes of Milano and Pavia to form a Consortium and realize the National Centre for Oncological Hadrontherapy in Milano. The Policlinico Ospedale Maggiore offered a site and on June 17, 1996, an instrument of understanding among the six institutions and TERA was signed.
As mentioned above, the status of CNAO
is described in the Blue Book and in the Addendum [11]. In summer
1996 a second one is in preparation. It describes the layout of
version D and it will be ready by October 1996.
The second project of the Hadrontherapy Programme, PACO, was started at the beginning of 1993. For about three years four working goups designed four different types of 200 MeV proton accelerators with the aim of eventually comparing their characteristics and costs. The work done is described in the recently printed "Green Book" [14].
As a starting point, the apparently vague notion of "compact accelerator and gantry" is quantified with the aim of reducing the cost of protontherapy. The national centre CNAO is designed as a centre of excellence where the best performances will be achieved for both protons and carbon ions. To reduce the costs, ions (and thus the treatment of radioresistant tumours) should be forgotten and "compact" accelerators should have somewhat more modest goals, of course without compromising the health care possibilities. It is obvious that striking such a balance is difficult and the conclusions are somewhat arbitrary. It is worthwhile reproducing the definition here because the adjective "compact" has been and still is misunderstood.
In the framework of the Hadrontherapy Programme, a proton facility for therapy deserves the adjective "compact" if:
(1) it accelerates a minimum of 2 ¥ 1010 protons per second to an energy of at least 200 MeV reliably and reproducibly, so to have a running efficiency close to the one of present conventional electron linacs (98%);
(2) it is possible to install the accelerator, the control room and the power supplies in a shielded bunker and a service area covering a total surface of less that 300 m2;
(3) it has a consumption - during irradiation - of less than 250 kW;
(4) costs, without buildings, not more than 17 million kLit (about 11 M$); this cost should include a rotating gantry with all the control and dose distribution systems, and should be able to run with both passive and active spreading systems;
(5) the addition of a second gantry should cost less than 3 million kLit (about 2 M$);
(6) a 70 MeV beam for eye therapy should also be foreseen and provided at low cost, if desired.
The required beam characterisics and field dimensions, not discussed here, are such that a "compact" accelerator can treat 85% of the about 5000 patienys/year treatable with the proton beams of CNAO.
The different solutions studied by the Hadrontherapy Collaboration for the "compact" proton acceleratoros are: a conventional synchrotron , a high-field synchroton, a high-frequency linac and a superconducting cyclotron. A room temperature cyclotron was not considered since IBA is producing it. Instead a chapter was written by P. Cohilis and Y. Jongen on a simplified and cheaper version of the IBA facility.
These five types of accelerators are described in five chapters of the Green Book. The remaining chapters are devoted to the status and future plans of the network RITA and of the three activities coordinated by the three Committees mentioned above. There is no space here to discuss these subjects.
The Green Book was prepared in connection
with the entering of Istituto Superiore di Sanità in the
field of hadrontherapy.
In fall 1993 the Physics Laboratory - directed by Prof. Martino Grandolfo - of Istituto Superiore di Sanità, which was since long active in the fields of proton radiobiology and dosimetry, decided to request special funds for the construction of a prototype of a "compact" accelerator (and its rotating gantry) and to finance R&D programmes in the fields of radiobiology, dosimetry, networking, pathology and treatment planning. This programme is now known as the TOP Project of ISS, where TOP stands for "Terapia Oncologica con Protoni". The initial funds (6'000'000 kLit) were allocated in 1994 and appropriated in 1995 with the understanding that about 80% of this sum had to be spent for the construction of a prototype of a "compact" accelerator, of a type yet to be decided. A contribution of 2'330'000 kLit was granted at the end of 1995. Requests for about the same total amount are pending.
In September 1995 draft copies of the
Green Book were distributed to the members of the Scientific Committee
of the TOP project. After auditing the persons responsible of
the various designs and considering the still limited funds available
(8'330'000 kLit) in December 1995 the Committee advised ISS to
concentrate on the construction of the first part of the high-frequency
linac [15], whose injector should also be capable of producing
PET isotopes. Istituto Superiore di Sanità accepted this
advice and in spring 1996 found a convenient site located in between
the buildings of ISS and of the oncological Institute Regina Elena.
After the decision of ISS the PACO project was terminated since
its mission was accomplished.
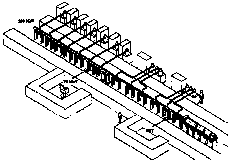
Fig. 3. The low current 3 GHz protontherapy
linac to be constructed in Rome by ISS, ENEA and TERA under the
direction of L. Picardi (ENEA).
The 3 GHz proton linac, designed in the framework of the Hadrontherapy Programme, solves the challange of accelerating low energy protons with a high frequency linac, in itself a scientifically interesting problem.
As shown in Fig. 5, the high frequency linac is made of three sections: a ten MeV injector of lower frequency (which can also produce PET isotopes), a new accelerating structure named Side Coupled Drift Tube Linac (SCDTL), which has been patented by ENEA, and a conventional Side Coupled Linac (SCL). Each SCDTL tank contains 5 or 6 drift tubes. about 1cm long with a 3-4 mm diameter hole. Permanent magnets located between adjacent tanks focus the accelerated beam. The SCDTL section accelerates protons to 70 MeV, and is the one financed at present. The SCL part will be built next and bring the beam to 200 MeV.
The overall TOP linac project is coordinated by Dr. Salvatore Frullani (ISS) and Dr. Luigi Picardi (ENEA) has been nominated responsible for the construction of the high-frequency linac. The new project was presented to the authorities and the public on June 24, 1996, during the Second National Day on Hadrontherapy, held in Rome in the auditorium of Institute Regina Elena.
The advantages of the high frequency
proton linac with respect to, for instance, a cyclotron are: (i)
the beam emittance is ten times smaller, so that the gantry can
be lighter and less expensive; (ii) the beam energy can be varied
continously between 140 and 200 MeV, as required by the tumour
depth; (iii) the accelerator has no problem of injection and extraction;
(iv) a linac is modular and can be constructed in pieces; (v)
when looked closely, the surface occupied by the centre is not
much larger than the one needed by a cyclotron.
With small modifications the SCL part of Fig. 3 can also be used as a booster of a 60-70 MeV proton cyclotron. A Section of the Green boook is devoted to this option, which is very interesting because in the world there are at least twenty 50-70 MeV cyclotrons which could be transformed in facilities for protontherapy of deep tumours. In the Green Book the study has been made with reference to the 62 MeV cyclotron of the Cyclotron Unit of the Clatterbridge Hospital (UK) [16]. In a total length of 13 m, 9 modules formed of 4 tanks and powered by 9 klystrons bring the proton beam from 62 to 200 MeV. The repetition rate is 400 Hz, which is good for a voxel active spreading of the beam. The overall linac capture efficiency, taking into account the fact that the linac acceptance is about three times the cyclotron emittance, is 1.5 10-4, so that the average proton current at 200 MeV is 10 nA. The plug power is about 100 kW. By switching off klystrons it is possible to vary the proton energy between 140 and 200 MeV.
The no-profit TERA Foundation is looking
at present for partners interested in transforming their cyclotrons
in a 200 MeV variable energy facility for protontherapy.
In Chapter 16 of the Green Book P.Chauvel and collaborators (Centre Antoine-Lacassagne, Cyclotron Biomedical, Nice) show that a protontherapy centre based on three gantries would be economically competitive with a centre of X-ray conformal radiotherapy (CRT), which use more than six cross-fired X-ray beams, if the accelerator and the gantries require investements not larger than the costs chosen in the definition of a "compact" accelerator: about 11 M$ for the accelerator and the first gantry, and about 2 M$ for each added gantry [17]. This would allow, taking into account both of the lifetime of the facility and of the cost of the staff, to charge about 1000-1100 DM for each 30 min session of protontherapy, so that a complete average treatment of 20 sessions would cost not more than 22'000 DM. This is a crucial point for the future developments of affordable hospital based protontherapy.
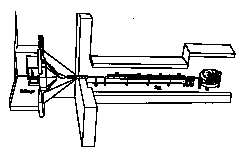
Fig. 4. The SCL part of the protontherapy
linac can be used as a booster for a 60-70 MeV cyclotron. The
parameters are given in Ref. 16.
To understand the meaning of this figures it is worth recalling that X-ray radiotherapy is the cheapest of all oncological therapies. In fact precise evaluations by G. Gademann [12] and by E. Borgonovi et al. of the Bocconi University [18] indicate that while a conventional radiotherapy costs between 6'000 and 7'000 DM, an average oncological surgery costs 15'000 DM and a heavy chemotherapy for a systemic tumour goes up to 60'000 DM. Recent calculations indicate that an average CRT with X-rays treament may cost 17'000-19'000 DM.
Comparing these figures with the ones
possible with a "compact" proton accelerator and gantry
system of the cost indicated above, and taking into account
the cost of the failures, Chauvel et al. conclude that protontherapy
is economically competitive with CRT. This argument represent
a strong incentive to develop cheap proton accelerator and gantries,
which do not require special buildings for the installation and
consume little plug power. In a few years we shall know wether
this interesting challange has been met.
I am very grateful to Philip Bryant,
Marco Pullia, Sandro Rossi and Mario Weiss for helping me in
preparing this review.
[1] W.H. Scharf and O.A. Chomicki, Physica Medica, 1996, in print.
[2] A. Wambersie, these Proceedings.
[3] R.Wilson, Radiology 47 (1946) 487.
[4] The statistics are published in Particles the journal of the Particle Therapy Coordination Group (PTCOG).
[5] H. Sato et al., EPAC94, V. Suller and Ch. Petit-Jean-Genaz Eds, World Scientific, p. 417.
[6] J.R. Castro, Hadrontherapy in Oncology, U. Amaldi and B. Larsson Eds, Elsevier, Amsterdam, 1994, p. 208.
[7] G. Coutrakon et al., Ref. 17, p. 282.
[9] E. Pedroni, Ref. 5, p. 407.
[10] G. Kraft et al., Ref. 6, p. 217.
[11] The TERA Project and the Centre for Oncological Hadrontherapy, Vol. I and Vol. II, U. Amaldi and M. Silari Eds, INFN, Frascati, 1995. Addendum, D. Campi and M. Silari Eds. The whole collection is called the "Blu Book".
[12] G. Gademann, Ref. 6, p. 59.
[13] Y. Jongen, Ref. 5, p. 355.
[14] The RITA Network and the Design of Compact Accelerators, U. Amaldi, M. Grandolfo and L. Picardi Eds, INFN, Frascati, 1996. The "Green Book".
[15] M. Weiss et al., Ref. 14, p. 213.
[16] M. Weiss et al., Ref. 14, p. 254.
[17] P. Chauvel et al., Ref. 14, p. 433.
[18] E. Borgonovi et al., Ref. 11, Vol. II, p. 535.